| 生物活性 | |||
|---|---|---|---|
| 靶点 |
|
||
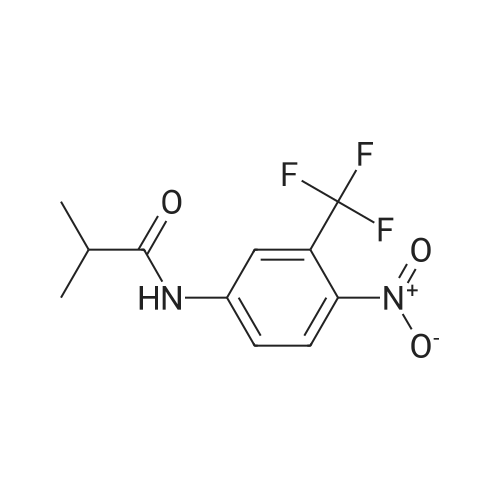
| 描述 | The androgen receptor (AR) and the androgen-AR signaling pathway play a significant role in male sexual differentiation and the development and function of male reproductive and non-reproductive organs [7]. Flutamide, mainly used in the treatment of prostate cancer, is an antiandrogenic drug. Flutamida-OH is the active metabolite form of Flutamide and directly binds to the androgen receptor with Ki of 55 nM. In rat adenohypophysial cells in primary culture, the specific uptake of [3H] testosterone (T) is completely blocked by increasing concentrations of the pure antiandrogen flutamide-OH at an IC50 value of 50 nM [8]. In adult male rats, Treatment for 10 days with flutamide (5 mg/rat, twice daily) caused a marked stimulation of plasma testosterone (T) associated with a significant increase in plasma gonadotropin concentrations and inhibited plasma prolactin (PRL) levels. Moreover, flutamide treatment alone produces an important inhibition of ventral prostate and seminal vesicle weights associated with a significant decrease in prostatic beta-adrenergic receptor levels [9]. | ||
| 实验方案 | |||
|---|---|---|---|
| 1mg | 5mg | 10mg | |
|
1 mM 5 mM 10 mM |
3.62mL 0.72mL 0.36mL |
18.10mL 3.62mL 1.81mL |
36.20mL 7.24mL 3.62mL |
| 参考文献 |
|---|