| 生物活性 | |||
|---|---|---|---|
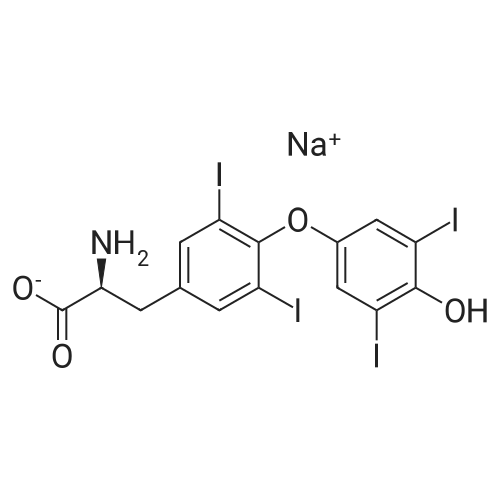
| 描述 | L-Thyroxine sodium (Levothyroxine sodium) is a synthetic hormone for the research of hypothyroidism[3]. The topic of pharmacological interference on L-T4 (L-thyroxine) therapy addresses the patient with primary hypothyroidism, in whom periodic measurement of serum thyrotropin (TSH) is the biochemical target[4]. Compared with L-T4 alone, replacement therapy with the combination of L-T4+L-T3 (L-triiodothyronine) shows favourable changes in serum lipid profile, but higher activation of bone resorption[5]. Standard L-thyroxine supplementation in HT (Hashimoto's thyroiditis) patients exerted significant immunoregulatory effects, associated with quantitative and phenotypic changes of peripheral blood DC (dendritic cells) subpopulations[6]. The thyroid hormone affects ventricular inhomogenicity, and that L-thyroxine replacement therapy may reduce malignant ventricular arrhythmia and sudden cardiac death in primary hypothyroidism[7]. | ||
| 临床研究 | |||||
|---|---|---|---|---|---|
| NCT号 | 适应症或疾病 | 临床期 | 招募状态 | 预计完成时间 | 地点 |
| NCT03693612 | Neoplasms | Phase 1 Phase 2 | Recruiting | June 16, 2022 | United States, Massachusetts ... 展开 >> GSK Investigational Site Not yet recruiting Boston, Massachusetts, United States, 02215 Contact: US GSK Clinical Trials Call Center 877-379-3718 GSKClinicalSupportHD@gsk.com Contact: EU GSK Clinical Trials Call Centre 877-379-3718 GSKClinicalSupportHD@gsk.com United States, New York GSK Investigational Site Not yet recruiting New York, New York, United States, 10016- Contact: US GSK Clinical Trials Call Center 877-379-3718 GSKClinicalSupportHD@gsk.com Contact: EU GSK Clinical Trials Call Centre 877-379-3718 GSKClinicalSupportHD@gsk.com GSK Investigational Site Not yet recruiting New York, New York, United States, 10032 Contact: US GSK Clinical Trials Call Center 877-379-3718 GSKClinicalSupportHD@gsk.com Contact: EU GSK Clinical Trials Call Centre 877-379-3718 GSKClinicalSupportHD@gsk.com United States, Oklahoma GSK Investigational Site Not yet recruiting Oklahoma City, Oklahoma, United States, 73104 Contact: US GSK Clinical Trials Call Center 877-379-3718 GSKClinicalSupportHD@gsk.com Contact: EU GSK Clinical Trials Call Centre 877-379-3718 GSKClinicalSupportHD@gsk.com United States, Pennsylvania GSK Investigational Site Not yet recruiting Pittsburgh, Pennsylvania, United States, 15232 Contact: US GSK Clinical Trials Call Center 877-379-3718 GSKClinicalSupportHD@gsk.com Contact: EU GSK Clinical Trials Call Centre 877-379-3718 GSKClinicalSupportHD@gsk.com United States, Texas GSK Investigational Site Recruiting San Antonio, Texas, United States, 78240 Contact: US GSK Clinical Trials Call Center 877-379-3718 GSKClinicalSupportHD@gsk.com Contact: EU GSK Clinical Trials Call Centre 877-379-3718 GSKClinicalSupportHD@gsk.com Principal Investigator: Anthony Tolcher Australia, New South Wales GSK Investigational Site Not yet recruiting Darlinghurst, New South Wales, Australia, 2010 Contact: US GSK Clinical Trials Call Center 877-379-3718 GSKClinicalSupportHD@gsk.com Contact: EU GSK Clinical Trials Call Centre 877-379-3718 GSKClinicalSupportHD@gsk.com Australia, Victoria GSK Investigational Site Not yet recruiting Melbourne, Victoria, Australia, 3000 Contact: US GSK Clinical Trials Call Center 877-379-3718 GSKClinicalSupportHD@gsk.com Contact: EU GSK Clinical Trials Call Centre 877-379-3718 GSKClinicalSupportHD@gsk.com Canada, Ontario GSK Investigational Site Not yet recruiting Ottawa, Ontario, Canada, K1H 8L Contact: US GSK Clinical Trials Call Center 877-379-3718 GSKClinicalSupportHD@gsk.com Contact: EU GSK Clinical Trials Call Centre 877-379-3718 GSKClinicalSupportHD@gsk.com GSK Investigational Site Not yet recruiting Toronto, Ontario, Canada, M5G 2M Contact: US GSK Clinical Trials Call Center 877-379-3718 GSKClinicalSupportHD@gsk.com Contact: EU GSK Clinical Trials Call Centre 877-379-3718 GSKClinicalSupportHD@gsk.com 收起 << |
| NCT00945282 | Infection, Human Immunodeficie... 展开 >>ncy Virus 收起 << | Phase 2 | Completed | - | Argentina ... 展开 >> GSK Investigational Site Buenos Aires, Argentina, B1602DBG 收起 << |
| NCT02705807 | Cardiovascular Disease | Phase 4 | Completed | - | Japan ... 展开 >> GSK Investigational Site Okayama, Japan, 701-1192 收起 << |
| 实验方案 | |||
|---|---|---|---|
| 1mg | 5mg | 10mg | |
|
1 mM 5 mM 10 mM |
1.25mL 0.25mL 0.13mL |
6.26mL 1.25mL 0.63mL |
12.52mL 2.50mL 1.25mL |
| 参考文献 |
|---|