| 生物活性 | |||
|---|---|---|---|
| 靶点 |
|
||
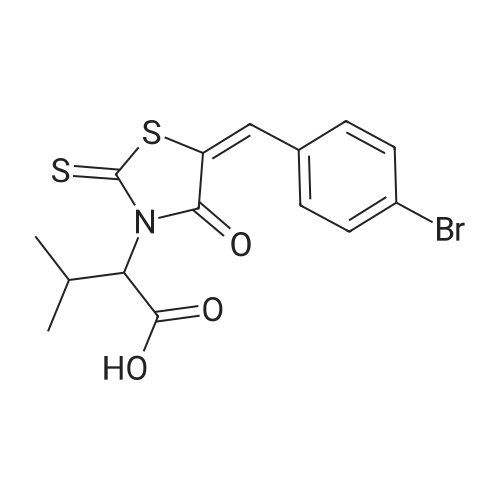
| 描述 | BH3 domain-mediated dimerization plays a key role in the regulation of pro-apoptotic and anti-apoptotic activities of the Bcl-2 family proteins. BH3I-1 is a small-molecule, cell-permeable inhibitor of the BH3-domain-mediated dimerization. The Ki values of BH3I-1 for the inhibition of Bak/Bcl-xL-His6 interaction determined by FP and NMR titration assays are 2.4±0.2 and 7.8±0.9μM, respectively. The addition of BH3I-1 (50 and 200μM) dose-dependently decreased the tBid binding in vitro. Treatment of JK cells with 100μM BH3I-1 induced DNA fragmentation and an increase in caspase-3-like and caspase-9-like activities. BH3I-1 at 100µM also induced cytochrome c release in HeLa cells at 48-h post-treatment. Treatment of JK/Bcl-xL cells with 100 µM BH3I-1 induced the appearance of sub-G1 DNA, indicative of apoptosis.[3] BH3I-1 at a dose of 50μM promoted the apoptosis of murine bone marrow-derived eosinophils and human eosinophils isolated from the peripheral blood of healthy subjects.[4] | ||
| 作用机制 | BH3I-1 inhibited BH3-domain-mediated dimerization by preventing BH3 domain-mediated interaction between Bcl-2 family members.[3] | ||
| 实验方案 | |||
|---|---|---|---|
| 1mg | 5mg | 10mg | |
|
1 mM 5 mM 10 mM |
2.50mL 0.50mL 0.25mL |
12.49mL 2.50mL 1.25mL |
24.98mL 5.00mL 2.50mL |
| 参考文献 |
|---|