| 生物活性 | |||
|---|---|---|---|
| 靶点 |
|
||
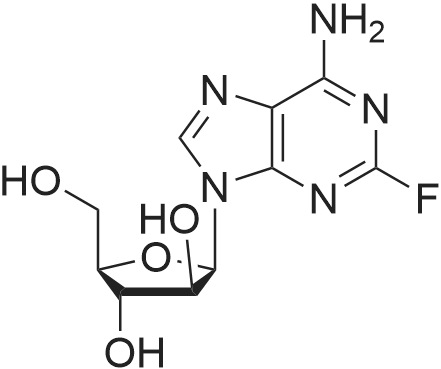
| 描述 | Fludarabine (2-F-ARAA, 2-fluoro ARA-A) is a chemotherapy drug used in the treatment of chronic lymphocytic leukemia (see https://www.fda.gov/) for targeting on DNA synthesis. It can also work as an inhibitor of STAT1 activation. The mechanism of Fludarabine action against to DNA synthesis is similar to other nucleotide analogues. Fludarabine can be phosphorylated to form as the Fludarabine triphosphate and then compete with natural nucleotides to inhibit DNA polymeraseα and incorporate into DNA strand. It can also act on ribonucleotide reductase and thus deplete the dATP pool[1]. Fludarabine can also cause a specific depletion of STAT1, but not of other STATs, as well as cytokine-induced activation of STAT1 and STAT1-dependent gene transcription in lymphocytes[2]. | ||
| 作用机制 | Fludarabine can inhibit DNA synthesis through targeting to ribonucleotide reductase and DNA polymerase. | ||
| 细胞研究 | |||||
|---|---|---|---|---|---|
| 细胞系 | 浓度 | 检测类型 | 检测时间 | 活性说明 | 数据源 |
| 624.38mel | 50 μM | Function Assay | 24 h | decreases IDO expression | 24911872 |
| 624.38mel | 50-200 μM | Function Assay | 24 h | inhibits IDO activity independently of mRNA levels | 24911872 |
| A549 | Growth Inhibition Assay | IC50=15.7±2.8 µM | 23377192 | ||
| 临床研究 | |||||
|---|---|---|---|---|---|
| NCT号 | 适应症或疾病 | 临床期 | 招募状态 | 预计完成时间 | 地点 |
| NCT00006251 | Acute Undifferentiated Leukemi... 展开 >>a Adult Nasal Type Extranodal NK/T-cell Lymphoma Anaplastic Large Cell Lymphoma Angioimmunoblastic T-cell Lymphoma Childhood Burkitt Lymphoma Childhood Diffuse Large Cell Lymphoma Childhood Grade III Lymphomatoid Granulomatosis Childhood Immunoblastic Large Cell Lymphoma Childhood Myelodysplastic Syndromes Childhood Nasal Type Extranodal NK/T-cell Lymphoma Chronic Myelomonocytic Leukemia Cutaneous B-cell Non-Hodgkin Lymphoma de Novo Myelodysplastic Syndromes Extranodal Marginal Zone B-cell Lymphoma of Mucosa-associated Lymphoid Tissue Hepatosplenic T-cell Lymphoma Intraocular Lymphoma Juvenile Myelomonocytic Leukemia Mast Cell Leukemia Myelodysplastic/Myeloproliferative Neoplasm, Unclassifiable Myeloid/NK-cell Acute Leukemia Nodal Marginal Zone B-cell Lymphoma Noncutaneous Extranodal Lymphoma Peripheral T-cell Lymphoma Post-transplant Lymphoproliferative Disorder Previously Treated Myelodysplastic Syndromes Primary Systemic Amyloidosis Recurrent Adult Acute Lymphoblastic Leukemia Recurrent Adult Acute Myeloid Leukemia Recurrent Adult Burkitt Lymphoma Recurrent Adult Diffuse Large Cell Lymphoma Recurrent Adult Diffuse Mixed Cell Lymphoma Recurrent Adult Diffuse Small Cleaved Cell Lymphoma Recurrent Adult Grade III Lymphomatoid Granulomatosis Recurrent Adult Hodgkin Lymphoma Recurrent Adult Immunoblastic Large Cell Lymphoma Recurrent Adult Lymphoblastic Lymphoma Recurrent Adult T-cell Leukemia/Lymphoma Recurrent Childhood Acute Lymphoblastic Leukemia Recurrent Childhood Acute Myeloid Leukemia Recurrent Childhood Anaplastic Large Cell Lymphoma Recurrent Childhood Grade III Lymphomatoid Granulomatosis Recurrent Childhood Large Cell Lymphoma Recurrent Childhood Lymphoblastic Lymphoma Recurrent Childhood Small Noncleaved Cell Lymphoma Recurrent Cutaneous T-cell Non-Hodgkin Lymphoma Recurrent Grade 1 Follicular Lymphoma Recurrent Grade 2 Follicular Lymphoma Recurrent Grade 3 Follicular Lymphoma Recurrent Mantle Cell Lymphoma Recurrent Marginal Zone Lymphoma Recurrent Mycosis Fungoides/Sezary Syndrome Recurrent Renal Cell Cancer Recurrent Small Lymphocytic Lymphoma Recurrent/Refractory Childhood Hodgkin Lymphoma Refractory Chronic Lymphocytic Leukemia Refractory Hairy Cell Leukemia Refractory Multiple Myeloma Small Intestine Lymphoma Splenic Marginal Zone Lymphoma Stage II Multiple Myeloma Stage III Multiple Myeloma T-cell Large Granular Lymphocyte Leukemia Testicular Lymphoma Waldenström Macroglobulinemia 收起 << | Phase 1 Phase 2 | Active, not recruiting | - | United States, Washington ... 展开 >> Fred Hutchinson Cancer Research Center/University of Washington Cancer Consortium Seattle, Washington, United States, 98109 Italy University of Torino Torino, Italy, 10126 收起 << |
| NCT01723839 | Chronic Lymphocytic Leukemia (... 展开 >>CLL) 收起 << | Phase 2 | Active, not recruiting | August 2018 | United States, New Jersey ... 展开 >> John Theurer Cancer Center at HackensackUMC Hackensack, New Jersey, United States, 07601 收起 << |
| NCT00544466 | Leukemia Myel... 展开 >>odysplastic Syndromes 收起 << | Phase 1 Phase 2 | Active, not recruiting | July 2019 | United States, California ... 展开 >> City of Hope Medical Center Duarte, California, United States, 91010-3000 收起 << |
| 实验方案 | |||
|---|---|---|---|
| 1mg | 5mg | 10mg | |
|
1 mM 5 mM 10 mM |
3.51mL 0.70mL 0.35mL |
17.53mL 3.51mL 1.75mL |
35.06mL 7.01mL 3.51mL |
| 参考文献 |
|---|