| 生物活性 | |||
|---|---|---|---|
| 靶点 |
|
||
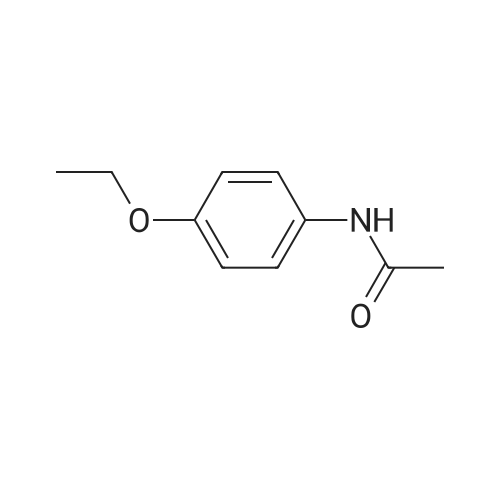
| 描述 | Phenacetin is a non-opioid analgesic without anti-inflammatory properties, inhibits COX-3 activity[3]. Phenacetin has been linked to renal papillary necrosis in human beings[4]. Since a major portion of a dose of phenacetin is rapidly metabolised to paracetamol, it seems possible that phenacetin owes some of its therapeutic activity to its main metabolite, paracetamol, whereas its most troublesome side effect (methaemoglobinaemia) is due to another metabolite, p-phenetidine[5]. Phenacetin treatment caused considerable nephrotoxicity and hepatotoxicity. Bioisosteric replacement of amide bond by 1,2,3-triazole in the phenacetin moiety yields conjugates with superior efficacy and diminished toxicity[6]. Combination analgesics containing phenacetin increase the risk of development of tumours in the renal pelvis. Phenacetin also increases the risk of development of cancer of the bladder[7]. | ||
| 实验方案 | |||
|---|---|---|---|
| 1mg | 5mg | 10mg | |
|
1 mM 5 mM 10 mM |
5.58mL 1.12mL 0.56mL |
27.90mL 5.58mL 2.79mL |
55.80mL 11.16mL 5.58mL |