| 生物活性 | |||
|---|---|---|---|
| 靶点 |
|
||
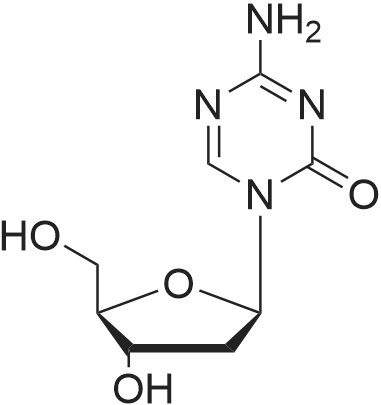
| 描述 | The DNA methyltransferase (DNMT) family are enzymes that can mediate the DNA methylation. Inhibiting the DNMTs have shown particular promise in reducing the formation of tumors[5]. The decitabine is a potent inhibitor of the DNA methylation with IC50 value of 100 and 10 ng/ml for 72 and 96h of exposure on both HL-60 and KG1a leukemic cells[6]. The human aortic smooth muscle cells (HASMCs) were treated with decitabine for 48 hours. The DNMT1 expression measured by western blotting assay in the nuclear fraction was reduced by the decitabine. The expression of the osteogenic genes ALP, Max2, BMP2, Pit-1, SM22 and -SMA in HASMCs measured by RT-qPCR was increased by decitabine[7]. The hyperoxia-indued lung injury Sprague-Dawley rats were injected with decitabine at a dose of 0.5 mg/kg/day for 14 days. The survival rate of the treatment group was significantly higher than the hyperoxia group. The P16 methylation rate in the lung tissues measured by PCR was induced by the hyperoxia and the decitabine could effectively reverse the hypermethylation of P16[8]. | ||
| 作用机制 | Decitabine could combine with DNA during DNA replication and form covalent complexes with the DNMT1. Therefore, the methyl transfer activity of the enzyme is inhibited[8]. | ||
| 细胞研究 | |||||
|---|---|---|---|---|---|
| 细胞系 | 浓度 | 检测类型 | 检测时间 | 活性说明 | 数据源 |
| A375 | 0.5 μM | Growth Inhibition Assay | 1/5/8 d | inhibits proliferation and induces differentiation of melanoma cells | 21796622 |
| A498 | 0.01-10μM | Apoptosis Assay | 72 h | induces synergistic responses with romidepsin | 22826467 |
| A549 | 5/10/20/50 μM | Apoptosis Assay | 48 h | inhibits the cell viability | 23582784 |
| 临床研究 | |||||
|---|---|---|---|---|---|
| NCT号 | 适应症或疾病 | 临床期 | 招募状态 | 预计完成时间 | 地点 |
| NCT02564536 | Chronic Myelomonocytic Leukemi... 展开 >>a Juvenile Myelomonocytic Leukemia Atypical Chronic Myeloid Leukemia Myeloproliferative Neoplasm Myelodysplastic Syndromes Myelofibrosis 收起 << | Phase 1 | Withdrawn(Lack of funding foll... 展开 >>owing full FDA clinical hold) 收起 << | June 30, 2020 | - |
| NCT01130506 | Previously Treated Myelodyspla... 展开 >>stic Syndrome Recurrent Adult Acute Myeloid Leukemia Refractory Acute Myeloid Leukemia Secondary Acute Myeloid Leukemia Therapy-Related Acute Myeloid Leukemia Untreated Adult Acute Myeloid Leukemia 收起 << | Phase 1 | Completed | - | United States, Ohio ... 展开 >> Ohio State University Comprehensive Cancer Center Columbus, Ohio, United States, 43210 United States, Texas M D Anderson Cancer Center Houston, Texas, United States, 77030 收起 << |
| NCT03240211 | PTCL CTCL | Phase 1 | Recruiting | December 2020 | United States, New York ... 展开 >> 51 West 51st Street, Suite 200 Recruiting New York, New York, United States, 10019 Contact: Michelle Malanga 212-326-5731 mm4629@cumc.columbia.edu Italy University of Bologna Not yet recruiting Bologna, Italy Principal Investigator: Pier Luigi Zinzani, MD, PhD Korea, Republic of Samsung Medical Center Not yet recruiting Seoul, Korea, Republic of Principal Investigator: Won Seog Kim, MD, PhD 收起 << |
| 实验方案 | |||
|---|---|---|---|
| 1mg | 5mg | 10mg | |
|
1 mM 5 mM 10 mM |
4.38mL 0.88mL 0.44mL |
21.91mL 4.38mL 2.19mL |
43.82mL 8.76mL 4.38mL |
| 参考文献 |
|---|