| 生物活性 | |||
|---|---|---|---|
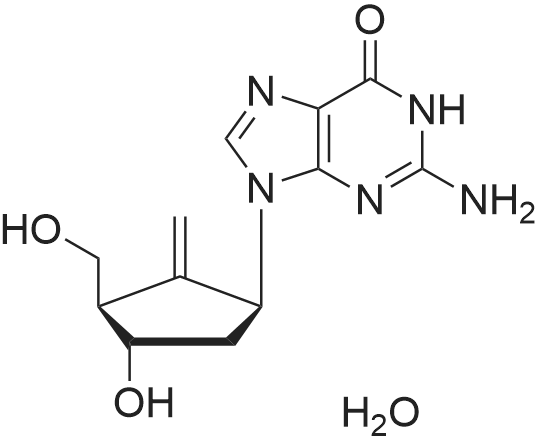
| 描述 | Entecavir (ETV) Monohydrate is a first-line therapy for chronic hepatitis B virus (HBV), demonstrating potent suppression of HBV DNA and a high barrier to viral resistance[3]. Entecavir, a new deoxyguanosine analog, represents a third agent within the nucleoside/nucleotide HBV polymerase inhibitor class with distinct advantages over lamivudine and adefovir dipivoxil: it has a three-step mechanism of action, is the most potent inhibitor of HBV DNA polymerase, is not associated with any major adverse effects, and has a limited potential for resistance. In phase II and III clinical trials, entecavir was found to be superior to lamivudine for all primary endpoints evaluated in both nucleoside-naïve and lamivudine-resistant patients. Entecavir was effective in both HBeAg-positive and HBeAg-negative nucleoside-naïve patients[4]. In chronic hepatitis B patients experiencing clevudine-induced myopathy, switching to entecavir 0.5 mg per day showed a resolution of myopathy and adequate viral suppression[5]. Entecavir (ETV) resistance of hepatitis B virus (HBV) conventionally requires rt184, 202, or 250 mutations plus lamivudine-resistance mutation (rtM204V/I ± L180M)[6]. | ||
| 实验方案 | |||
|---|---|---|---|
| 1mg | 5mg | 10mg | |
|
1 mM 5 mM 10 mM |
3.39mL 0.68mL 0.34mL |
16.93mL 3.39mL 1.69mL |
33.87mL 6.77mL 3.39mL |
| 参考文献 |
|---|