| 生物活性 | |||
|---|---|---|---|
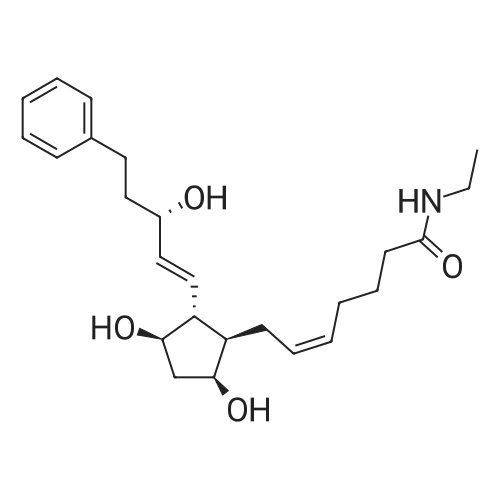
| 描述 | Bimatoprost, a synthetic prostamide F2α analog originally approved for the treatment of ocular hypertension and open-angle glaucoma, is now FDA approved as a 0.03%, solution to be applied once daily to increase eyelashes growth[3]. In March 2020, bimatoprost implant received its first approval, in the USA, for use to reduce intraocular pressure (IOP) in patient with open angle glaucoma or ocular hypertension[4]. | ||
| 实验方案 | |||
|---|---|---|---|
| 1mg | 5mg | 10mg | |
|
1 mM 5 mM 10 mM |
2.41mL 0.48mL 0.24mL |
12.03mL 2.41mL 1.20mL |
24.06mL 4.81mL 2.41mL |
| 参考文献 |
|---|