| 生物活性 | |||
|---|---|---|---|
| 靶点 |
|
||
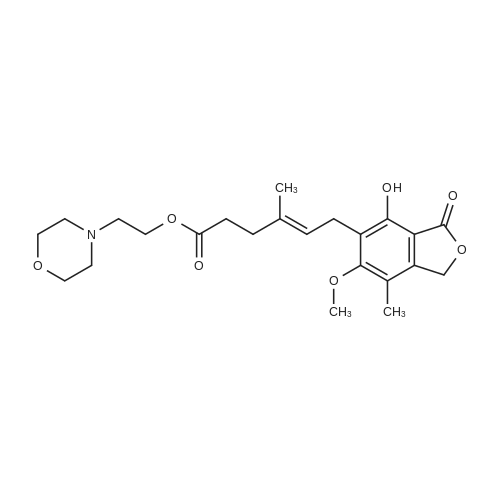
| 描述 | Mycophenolate mofetil (MMF) is a relatively new systemic immunosuppressive agent used in the field of dermatologic[3]. It is the morpholinoethylester prodrug of mycophenolic acid, an agent that inhibits the proliferation of B and T lymphocytes through noncompetitive, reversible inhibition of inosine monophosphate dehydrogenase, a key enzyme in the de novo synthetic pathway of guanine nucleotides. Mycophenolate mofetil is approved for the prevention of acute renal allograft rejection when given in combination with cyclosporine and steroids[4]. A phase I clinical trial showed MMF was well tolerated in renal transplant patients at doses up to 3,500 mg/day for up to two years. There was no correlation between the incidence of adverse effects and dose of MMF, and no overt nephrotoxicity, hepatotoxicity, or myelotoxicity was observed. In a large multicenter trial, MMF in combination with cyclosporine and prednisone was superior to a standard immunosuppressive regimen including azathioprine[5]. | ||
| 实验方案 | |||
|---|---|---|---|
| 1mg | 5mg | 10mg | |
|
1 mM 5 mM 10 mM |
2.31mL 0.46mL 0.23mL |
11.53mL 2.31mL 1.15mL |
23.07mL 4.61mL 2.31mL |
| 参考文献 |
|---|
|
[3]Zwerner J, Fiorentino D. Mycophenolate mofetil. Dermatol Ther. 2007 Jul-Aug;20(4):229-38 [5]Sollinger HW. Mycophenolate mofetil. Kidney Int Suppl. 1995 Dec;52:S14-7 |