| 生物活性 | |||
|---|---|---|---|
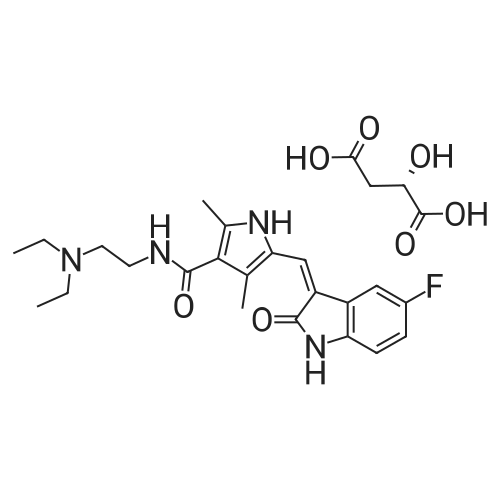
| 描述 | VEGFR (vascular endothelial growth factor) and PDGFR (platelet-derived growth factor receptor) are critical roles in tumor growth and suvival via autocrine and paracrine loops, making them the well validated targets for the treatment of cancers. Sunitinib Malate is the Malate form of Sunitinib. Sunitinib is a multiple RTKs inhibitor with IC50 values of 2nM and 80nM for VEGFR2 and PDGFRβ (measured by kinase activity)[1], respectively, also shows inhibition against KIT and FLT3 receptor[2]. The cellular kinase activity induced by VEGF/PDGF can be inhibited by Sunitinib with IC50 value of 5-50nM/10nM in 3T3 cells, while the PDGF-induced cell growth can be inhibited by Sunitinib with IC50 of 8nM[1]. Daily oral administration of Sunitinib at dose of 80mg/kg reduced growth of established SF763T tumor xenografts in athymic mice, as well as suppressed Colo205 tumor growth. Consistent with the cellular kinase study, the inhibition by Sunitinib against p-PDGFRβ can be observed in tumor after a single dose at 80mg/kg in mice bearing SF767T tumors and mice bearing Colo205 tumors, as well as suppressed p-FLK1 in A375 xenograft mice dosed 40mg/kg Sunitinib[2]. | ||
| 作用机制 | Sunitinib is an ATP-competitive multitargeted tyrosine kinase inhibitor.[3] | ||
| 细胞研究 | |||||
|---|---|---|---|---|---|
| 细胞系 | 浓度 | 检测类型 | 检测时间 | 活性说明 | 数据源 |
| 3T3 | Kinase Assay | Inhibition of PDGF-induced BrdU incorporation with IC50 of 0.007 μM | 12646019 | ||
| 3T3 | Growth Inhibition Assay | Inhibition of Platelet-derived growth factor induced 3T3 cell proliferation with IC50 of 0.01 μM | 12646019 | ||
| 3T3 | Function Assay | Inhibition of Vascular endothelial growth factor receptor with IC50 of 0.05 μM | 12646019 | ||
| 临床研究 | |||||
|---|---|---|---|---|---|
| NCT号 | 适应症或疾病 | 临床期 | 招募状态 | 预计完成时间 | 地点 |
| NCT00663559 | Carcinoma Renal Cells | Phase 2 | Completed | - | Spain ... 展开 >> Hospital Central de Asturias Oviedo, Asturias, Spain, 33006 Hospital Parc Taulí Sabadell, Barcelona, Spain, 08208 Hospital CLINIC Barcelona, Spain, 08036 Hospital Reina Sofía Córdoba, Spain, 14004 Hospital de Jaén Jaén, Spain, 23007 Hospital Clínico San Carlos Madrid, Spain, 28040 Hospital lozano Blesa Zaragoza, Spain, 50009 收起 << |
| NCT03097601 | - | Active, not recruiting | December 30, 2019 | France ... 展开 >> Centre Antoine LACASSAGNE Nice, France, 06189 收起 << | |
| NCT01243359 | Clear Cell Renal Cell Carcinom... 展开 >>a Recurrent Renal Cell Cancer Stage I Renal Cell Cancer Stage II Renal Cell Cancer Stage III Renal Cell Cancer Stage IV Renal Cell Cancer Unspecified Adult Solid Tumor, Protocol Specific 收起 << | Phase 1 | Completed | - | United States, Maryland ... 展开 >> Johns Hopkins University/Sidney Kimmel Cancer Center Baltimore, Maryland, United States, 21287 United States, Wisconsin University of Wisconsin Hospital and Clinics Madison, Wisconsin, United States, 53792 收起 << |
| 实验方案 | |||
|---|---|---|---|
| 1mg | 5mg | 10mg | |
|
1 mM 5 mM 10 mM |
1.88mL 0.38mL 0.19mL |
9.39mL 1.88mL 0.94mL |
18.78mL 3.76mL 1.88mL |
| 参考文献 |
|---|