| 生物活性 | |||
|---|---|---|---|
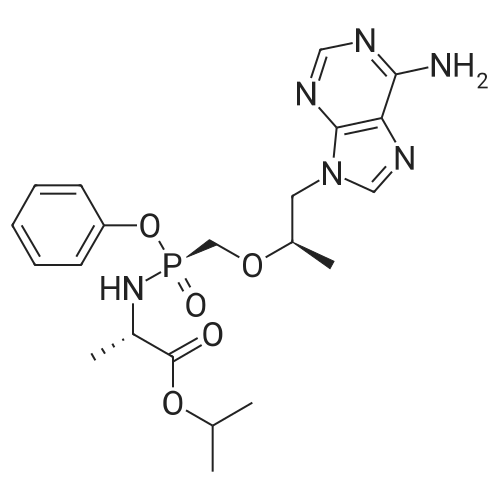
| 描述 | Reverse transcription and integration are the defining features of the Retroviridae; the common name "retrovirus" derives from the fact that these viruses use a virally encoded enzyme, reverse transcriptase (RT), to convert their RNA genomes into DNA[3]. GS-7340 (Tenofovir alafenamide) is an investigational oral prodrug of Tenofovir. Tenofovir is a HIV-1 nucleotide reverse transcriptase inhibitor. Tenofovir alafenamide (GS-7340) hemifumarate is an amidate prodrug of Tenofovir with good oral bioavailability and increases plasma stability compared to Tenofovir disoproxil fumarate (TDF)[4]. GS-7340 antiviral activities are similar across all cell types, ranging from 5 to 7 nM. The antiviral activity of TAF (Tenofovir alafenamide) is evaluated against a panel of HIV-1 and HIV-2 isolates, including HIV-1 group M subtypes A to G, as well as group N and O isolates. Overall, for the 29 primary HIV-1 isolates tested in PBMCs, TAF EC50s range from 0.1 to 12 nM, with a mean EC50 of 3.5 nM compared to a mean EC50 of 11.8 nM for AZT, which is used as an internal control. For the HIV-2 isolates, the mean EC50s are 1.8 nM for TAF and 6.4 nM for AZT[5]. GS-7340 is less nephrotoxic than its predecessor prodrug, tenofovir disoproxil fumarate (TDF). GS-7340's unique pharmacokinetic profile enables provision of lower required doses for antiviral efficacy. Lower concentrations reach renal tubules minimizing intracellular accumulation and mitochondrial damage[6]. | ||
| 细胞研究 | |||||
|---|---|---|---|---|---|
| 细胞系 | 浓度 | 检测类型 | 检测时间 | 活性说明 | 数据源 |
| MT2 cells | Cytotoxicity assay | Cytotoxicity against human MT2 cells, CC50=40 μM | 24686012 | ||
| 临床研究 | |||||
|---|---|---|---|---|---|
| NCT号 | 适应症或疾病 | 临床期 | 招募状态 | 预计完成时间 | 地点 |
| NCT03377608 | - | Completed | - | Uganda ... 展开 >> MU-JHU Care Ltd Kampala, Uganda 收起 << | |
| NCT02957864 | Renal Insufficiency,Chronic ... 展开 >> Hiv Therapeutic Agent Toxicity 收起 << | Phase 4 | Recruiting | February 2020 | Netherlands ... 展开 >> Ziekenhuis Rijnstate Recruiting Arnhem, Gelderland, Netherlands Contact: Marc Claassen, PhD MC Slotervaart Recruiting Amsterdam, Netherlands, 1066EC Contact: Saskia Vrouenraets, MD, PhD 0205129333 saskia.vrouenraets@slz.nl OLVG Not yet recruiting Amsterdam, Netherlands, 1091AC Contact: Guido van den Berk, MD, PhD 0205999111 g.e.l.vandenberk@olvg.nl Erasmus MC Recruiting Rotterdam, Netherlands, 3000CA Contact: ingeborg wijting, MD 0031107040704 i.wijting@erasmusmc.nl Contact: bart rijnders, MD PhD 0031107033510 b.rijnders@erasmusmc.nl Maasstad ziekenhuis Recruiting Rotterdam, Netherlands, 3079DZ Contact: Anna Roukens, MD, PhD 0102911911 roukensa@maasstadziekenhuis.nl 收起 << |
| NCT00924898 | Acute HIV Infection ... 展开 >> HIV Infections 收起 << | Phase 4 | Completed | - | United States, North Carolina ... 展开 >> The University of North Carolina - Chapel Hill Chapel Hill, North Carolina, United States, 27599 Duke University Durham, North Carolina, United States, 27707 收起 << |
| 实验方案 | |||
|---|---|---|---|
| 1mg | 5mg | 10mg | |
|
1 mM 5 mM 10 mM |
2.10mL 0.42mL 0.21mL |
10.49mL 2.10mL 1.05mL |
20.99mL 4.20mL 2.10mL |
| 参考文献 |
|---|