| 生物活性 | |||
|---|---|---|---|
| 靶点 |
|
||
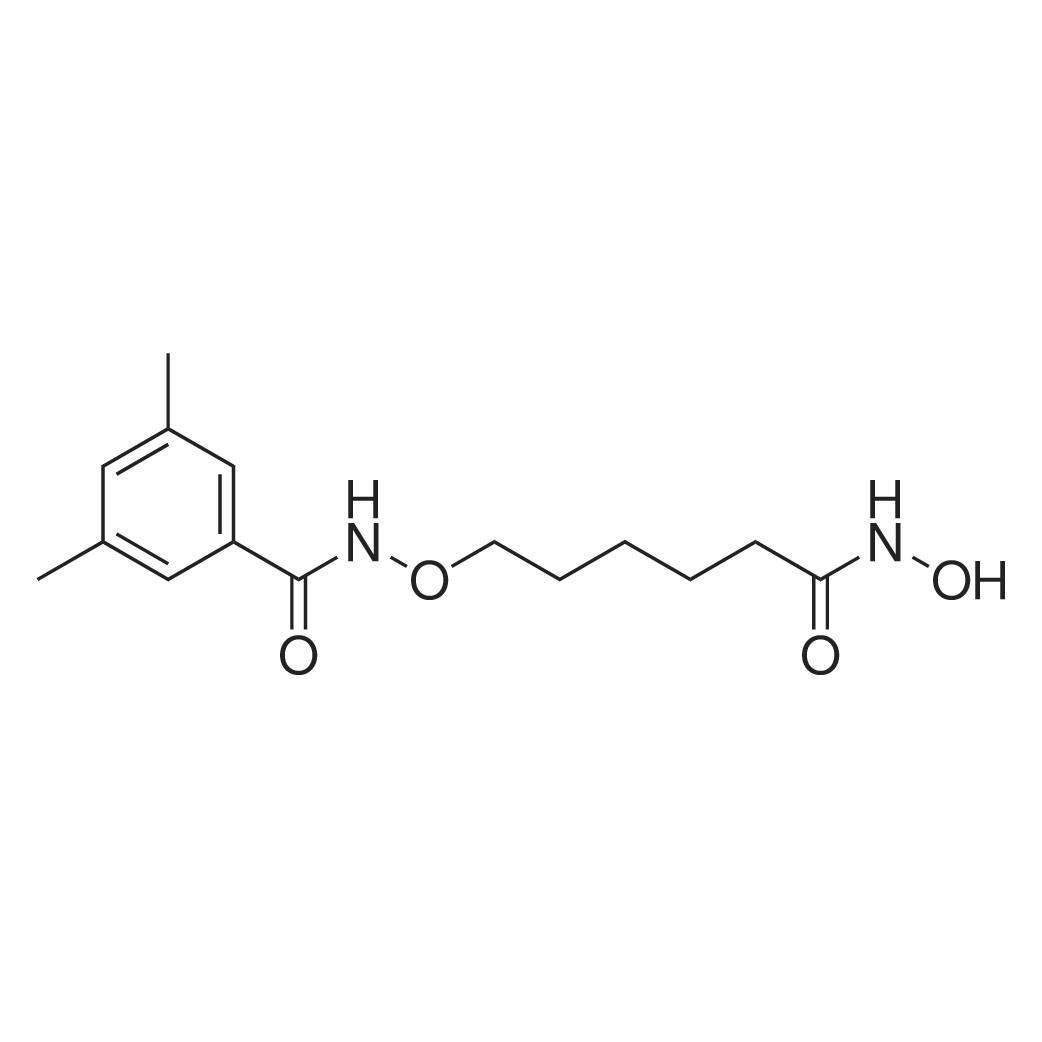
| 描述 | LMK235 a potent hydroxamate-based HDAC inhibitor with IC50 values of 320, 881, 11.9, 4.22, 55.7, 1278 and 852nM for HDAC1, 2, 4, 5, 6, 8 and 11, respectively. LMK235 inhibited HDAC activity of sensitive/Cisplatin-resistent MDA-MB-231, Cal27 and Kyse510 cells with IC50 values of 0.46μM/0.41μM, 0.36μM/0.36μM and 1μM /0.35μM, respectively, and exhibited cell growth inhibitory effect on sensitive/Cisplatin-resistent MDA-MB-231, Cal27 and Kyse510 cells with IC50 values of 1.37μM/1.68μM, 1.03μM/1.81μM and 2.96μM and 2.48μM, respectively. Compared with vorinostat, LMK235 showed similar effects on inhibition of cellular HDACs, but enhanced cytotoxic effects against the human cancer cell lines A2780, Cal27, Kyse510, and MDA-MB231. LMK-235 also showed nanomolar inhibition of HDAC4 and HDAC5, whereas vorinostat and TSA inhibit HDAC4 and HDAC5 in the higher micromolar range[2]. | ||
| 作用机制 | LMK235 is a hydroxamate-based HDAC inhibitor which can chelate the catalytic zinc ion of HDAC. The alkoxyamide connecting unit linker region contributes to the selectivity of it.[2] | ||
| 细胞研究 | |||||
|---|---|---|---|---|---|
| 细胞系 | 浓度 | 检测类型 | 检测时间 | 活性说明 | 数据源 |
| human A2780 cells | Cytotoxicity assay | 72 h | Cytotoxicity against cisplatin resistant human A2780 cells after 72 hrs by MTT assay, IC50=0.32 μM | 23252603 | |
| human A2780 cells | Cytotoxicity assay | 72 h | Cytotoxicity against human A2780 cells after 72 hrs by MTT assay, IC50=0.49 μM | 23252603 | |
| human A2780 cells | Cytotoxicity assay | 18 h | Inhibition of HDAC in human A2780 cells after 18 hrs by fluorescence assay, IC50=0.65 μM | 23252603 | |
| 实验方案 | |||
|---|---|---|---|
| 1mg | 5mg | 10mg | |
|
1 mM 5 mM 10 mM |
3.40mL 0.68mL 0.34mL |
16.99mL 3.40mL 1.70mL |
33.97mL 6.79mL 3.40mL |
| 参考文献 |
|---|