| 生物活性 | |||
|---|---|---|---|
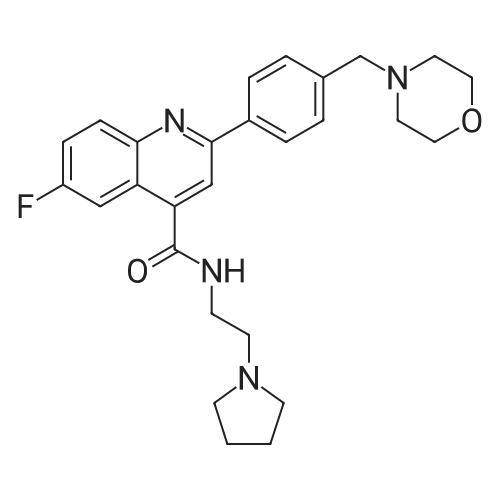
| 描述 | DDD107498 is a potent and novel spectrum of antimalarial activity against multiple life-cycle stages of the Plasmodium parasite, targeting on PfeEF2 (P. falciparum translation elongation factor 2), which is responsible for the GTP-dependent translocation of the ribosome along messenger RNA, and is essential for protein synthesis. DDD107498 showed excellent activity against 3D7 parasites with EC50 value of 1nM. DDD107498 was more potent than artesunate in ex vivo assays against a range of clinical isolates of both P. falciparum with median EC50 value of 0.81nM and P. vivax with median EC50 value of 0.51nM. DDD107498 displayed excellent pharmacokinetic properties in preclinical species, including good oral bioavailability and long plasma half-life. DDD107498 was very active in several mouse models of malaria. It had a 90% reduction in parasitaemia (ED90) of 0.57mg/kg after a single oral dose in mice infected with the rodent parasite Plasmodium berghei. DDD107498 at concentration<1μM dose-dependently protein synthesis via eEF2, in the cytoplasm as opposed to the apicoplast, the site of action of tetracycline and azithromycin[3]. | ||
| 作用机制 | DDD107498 targets protein synthesis via PfeEF2.[3] | ||
| 实验方案 | |||
|---|---|---|---|
| 1mg | 5mg | 10mg | |
|
1 mM 5 mM 10 mM |
2.16mL 0.43mL 0.22mL |
10.81mL 2.16mL 1.08mL |
21.62mL 4.32mL 2.16mL |
| 参考文献 |
|---|