| 生物活性 | |||
|---|---|---|---|
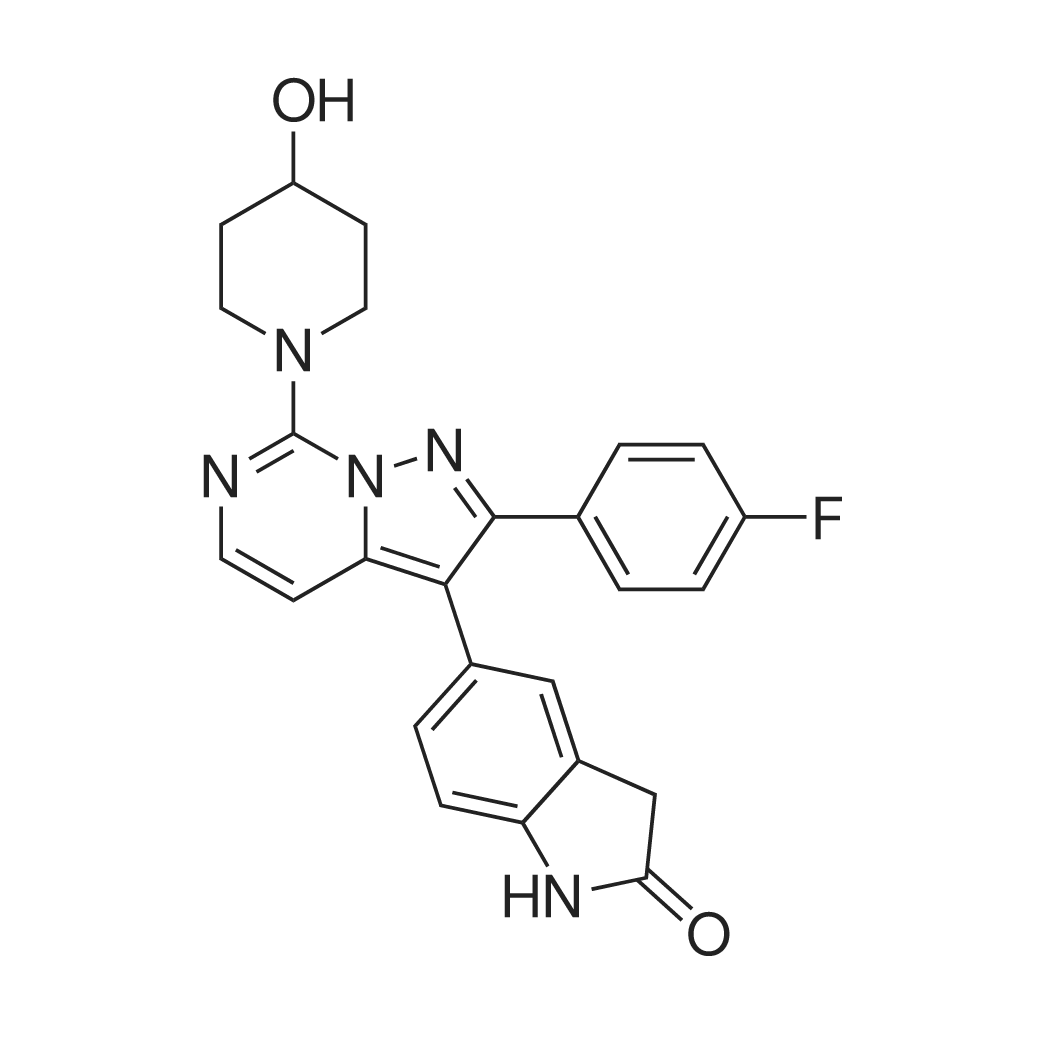
| 描述 | AMPA receptors (AMPARs) are assembled as tetramers of pore-forming subunits (GluA1−GluA4) together with a host of accessory proteins that work in concert to regulate receptor trafficking and pharmacology. Most, if not all, AMPARs are associated with transmembrane AMPAR regulatory proteins (TARPs), which are classified by sequence homology and function as Type I (γ-2, γ-3, γ-4, γ-8) or Type II (γ-5, γ-7)[2]. JNJ-61432059 is an oral active and selective negative modulator of AMPAR associated with trans-membrane AMPAR regulatory protein (TARP) γ-8. In a FLIPR assay using HEK-293 cells expressing a human GluA1o-γ-8 fusion construct, JNJ-61432059 has a pIC50 value of 9.7 for GluA1/γ-8[2]. JNJ-61432059 showed inhibition of CYPs 2C8 and 2C9 at lower concentrations (IC50s = 3.0 and 1.9 μM, respectively)[2]. After oral dosing at 10 mg/kg in rats, JNJ-61432059 distributed into the brain (Kpu,u = 0.4) despite low plasma exposures (Cmax = 26 ng/ml) and high clearance (Cl = 57 ml/min/kg). When JNJ-61432059 was incubated with hepatocytes for 4 h at 37 °C, the O-glucuronide was detected as the major metabolite in rat. When administered orally at 10 mg/kg, JNJ-61432059 showed high target engagement in mouse hippocampus, as measured by ex vivo autoradiography, with maximal receptor occupancy exceeding 90% at 1 h. The plasma and brain exposures at Tmax were highly linear over a wide dose range, and the receptor occupancy was well described by a Hill function with ED50 = 2.9 ± 0.7 mg/kg[2]. | ||
| 实验方案 | |||
|---|---|---|---|
| 1mg | 5mg | 10mg | |
|
1 mM 5 mM 10 mM |
2.25mL 0.45mL 0.23mL |
11.27mL 2.25mL 1.13mL |
22.55mL 4.51mL 2.25mL |
| 参考文献 |
|---|