| 生物活性 | |||
|---|---|---|---|
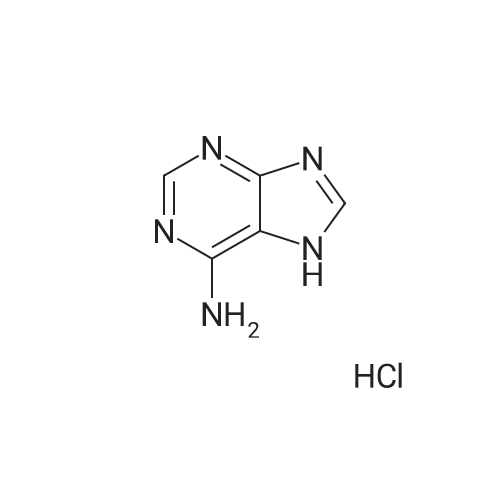
| 描述 | Adenine (6-Aminopurine), a purine, is one of the four nucleobases in the nucleic acid of DNA, with the other three being cytosine (C), guanine (G), and thymine (T). Adenine plays an important role in biochemistry, including cellular respiration and protein synethesis[1]. Cleave DNA preferentially at guanines, at adenines, at cytosines and thymines equally, and at cytosines alone. When the products of these four reactions are resolved by size, by electrophoresis on a polyacrylamide gel, the DNA sequence can be read from the pattern of radioactive bands[2]. Purine formation requires tautomerization of 5 to the conjugated amidine 6 (via hydrogen-tunneling, thermally with H(+) -catalysis, or by photolysis) or to keteneimine 7 (by photolysis)[3]. The adenine-uracil interaction, which involves two hydrogen bonds (rather than three, as in guanine-cytosine pairing) is weak and nonspecific. Pairing of adenine with many other partners has been observed with monomers, synthetic oligonucleotides and in RNA[4]. In addition to DNA cytosine methylation (5-methyl-2'-deoxycytidine, m5dC), DNA adenine methylation (N6-methyl-2'-deoxyadenosine, m6dA) is another DNA modification that has been discovered in eukaryotes[5]. | ||
| 实验方案 | |||
|---|---|---|---|
| 1mg | 5mg | 10mg | |
|
1 mM 5 mM 10 mM |
5.83mL 1.17mL 0.58mL |
29.14mL 5.83mL 2.91mL |
58.28mL 11.66mL 5.83mL |
| 参考文献 |
|---|