| 生物活性 | |||
|---|---|---|---|
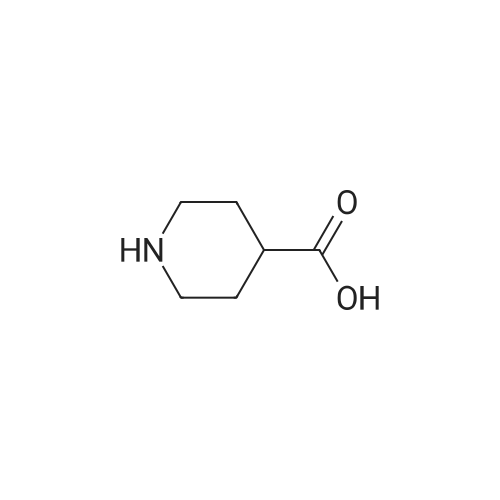
| 描述 | Isonipecotic acid was less potent than its unsaturated analogue isoguvacine as a GABA-mimetic and as an inhibitor of GABA binding[2]. The depolarizing action of all 4 analogues muscimol, isonipecotic acid, isoguvacine and N-methyl isoguvacine could be selectively antagonized by bicuculline methochloride and isopropyl bicyclophosphate. The potency of isoguvacine was 0.23 +/- 0.026 and isonipecotic acid 0.011 +/- 0.0028[3]. Modifications of the isonipecotic acid fragment of SNS-032 results in analogs which are more permeable and lower effluxed than SNS-032[4]. The most significant activity was demonstrated by the p-nitrophenyl esters of nipecotic and isonipecotic acids against bicuculline-induced seizures[5]. In vivo tests revealed statistically significant antinociceptive properties of isonipecotic acid (10 and 30 mg/kg), R-nipecotic acid (30 and 100 mg/kg) and S-nipecotic acid (100 mg/kg) in mice[6]. | ||
| 实验方案 | |||
|---|---|---|---|
| 1mg | 5mg | 10mg | |
|
1 mM 5 mM 10 mM |
7.74mL 1.55mL 0.77mL |
38.71mL 7.74mL 3.87mL |
77.42mL 15.48mL 7.74mL |
| 参考文献 |
|---|