| 生物活性 | |||
|---|---|---|---|
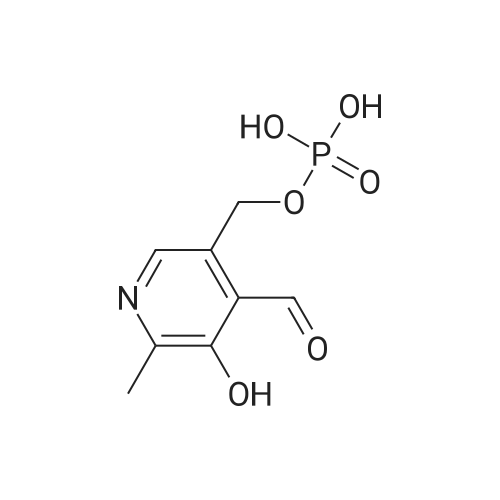
| 描述 | Pyridoxal phosphate is the active form of vitamin B6, acts as an inhibitor of reverse transcriptases, and is used for the treatment of tardive dyskinesia. Pyridoxal phosphate is biosynthesized de novo by two different pathways (the DXP dependent pathway and the R5P pathway) and can also be salvaged from the environment. It is also known to function as a singlet oxygen scavenger and has protective effects against oxidative stress in fungi[1]. Increasing Mg2+ concentration up to 14 mM overcame the suppression of self-splicing by pyridoxal phosphate up to 95% of the level of normal splicing, implying its interference with effective catalysis of Mg2+. The kinetic analysis demonstrated that pyridoxal phosphate acts as a mixed type noncompetitive inhibitor for the td intron RNA with a K(i) of 11.8 mM[2]. Pyridoxal phosphate-conjugated NEIL2 shows much lower activity than the intact enzyme over a wide range of enzyme/substrate ratios. After Pyridoxal phosphate conjugation, the ability of NEIL2 to bind the THF-ligand is completely lost[3]. | ||
| 实验方案 | |||
|---|---|---|---|
| 1mg | 5mg | 10mg | |
|
1 mM 5 mM 10 mM |
4.05mL 0.81mL 0.40mL |
20.23mL 4.05mL 2.02mL |
40.46mL 8.09mL 4.05mL |
| 参考文献 |
|---|